image:
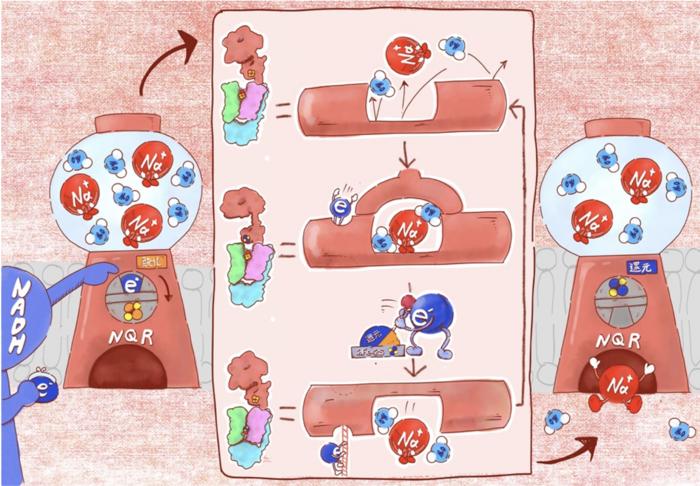
Overall image summarizing the findings of this study.
view more
Credit: Moe Ishikawa-Fukuda
Kyoto, Japan — The enzyme Na⁺-NQR is a sodium pump that drives the respiration of many marine and pathogenic bacteria. Using redox reactions, the process of exchanging electrons between materials, it powers the transportation of sodium ions across the membrane, supporting the growth of the bacteria.
Yet there is a mystery behind this mechanism, as scientists have had trouble understanding exactly how the redox reactions are linked to sodium-pumping. In particular, the lack of structural information on the key intermediate states that form while the enzyme is operating has posed a major challenge; determining these structures is essential to understanding how the pump functions.
This gap in knowledge motivated a team of researchers at Kyoto University to investigate what powers this mysterious sodium pump. Using cryo-electron microscopy performed by co-first author Moe Ishikawa-Fukuda, the team was able to directly capture multiple intermediate structural states of the enzyme as it transformed during operation. They then combined these structural snapshots with molecular dynamics simulations performed by co-first author Takehito Seki.
The simulations revealed that the sodium pump changes its structure in response to electron transfer inside the protein. These changes then drive sodium ion translocation across the bacterial cell membrane by opening and closing a gate within the membrane, allowing sodium ions to pass through.
“Our study is the first to clearly explain how redox reactions directly drive sodium ion transport at the molecular level, providing a new framework for understanding energy conversion in bacteria,” says Ishikawa-Fukuda.
The researchers were also surprised to discover the importance of a specific inhibitor called korormicin, which the team had identified and described in previous research. This compound plays a pivotal role in capturing critical intermediate states of the enzyme that are otherwise difficult to observe.
“Understanding redox-driven sodium pumping addresses a long-standing question in bioenergetics, revealing a strategy that is fundamentally different from the proton pump found in mammalian mitochondria,” says Seki.
Next, the team plans to investigate whether the structural states they identified can be targeted to selectively block operation of the sodium pump. This may open a door to the development of antibiotics that act on previously unexplored targets.
“Our goal was to understand how this sodium pump works at a fundamental level,” says team leader Masatoshi Murai. “Although this is basic research, we hope that clarifying these mechanisms will eventually contribute to the development of new strategies to combat pathogenic bacteria.”
###
The paper “The redox driven Na+-pumping mechanism in Vibrio cholerae NADH-quinone oxidoreductase relies on dynamic conformational changes” appeared on 12 February 2026 in Nature Communications, with doi: 10.1038/s41467-026-69182-w
This work is a collaboration with Rensselaer Polytechnic Institute researchers and is supported by an NIH grant. Team members also include researchers from the Kyoto Institute of Technology and the Institute for Molecular Science.
About Kyoto University
Kyoto University is one of Japan and Asia’s premier research institutions, founded in 1897 and responsible for producing numerous Nobel laureates and winners of other prestigious international prizes. A broad curriculum across the arts and sciences at undergraduate and graduate levels complements several research centers, facilities, and offices around Japan and the world. For more information, please see: http://www.kyoto-u.ac.jp/en
Journal
Nature Communications
Method of Research
Observational study
Subject of Research
Cells
Article Title
The redox driven Na+-pumping mechanism in Vibrio cholerae NADH-quinone oxidoreductase relies on dynamic conformational changes
Article Publication Date
12-Feb-2026
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

AloJapan.com