image:
Enantioselective Heterocoupling of 2-Naphthylamines with 2-Naphthol Derivatives via Cooperative Photoactivation and Chiral Vanadium(V) Catalysis
view more
Credit: The University of Osaka
A research group led by The University of Osaka has achieved a world-first in catalytic asymmetric synthesis, developing an innovative method for efficiently producing NOBIN, a valuable molecule used in pharmaceuticals. Their approach combines a vanadium catalyst, LED light, and oxygen, drastically reducing waste by eliminating byproduct formation common in conventional methods, and establishing a highly sustainable synthetic pathway.
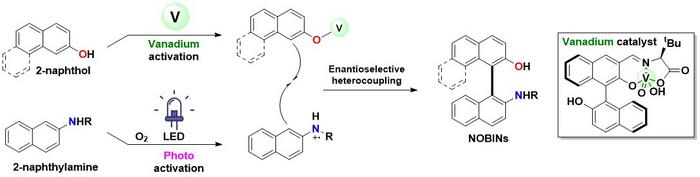
Many modern medicines and functional materials depend on molecules that come in “right-” and “left-handed” forms, known as chiral molecules. Traditionally, making these molecules requires multiple steps and often produces unwanted chemical waste. In the case of NOBIN, previous methods always produced additional unwanted byproducts, reducing efficiency and increasing environmental burden.
The team’s innovation lies in cooperatively combining a vanadium catalyst and light. The catalyst selectively converts 2-naphthol into a radical species. Concurrently, LED light under oxygen generates a cationic radical species from 2-naphthylamine via a charge-transfer complex. These two radicals then efficiently couple, exclusively yielding NOBIN derivatives. This allows for an ideal 1:1 input ratio of starting materials and utilizes low-energy LED light, significantly minimizing environmental impact and making the synthesis highly sustainable.
This clean process yields only water as a byproduct, showcasing exceptional environmental compatibility and waste reduction. Activating molecules using light is energy-saving and safe, accelerating next-generation asymmetric synthesis research. Professor Shinobu Takizawa, senior author of the study states, “This achievement opens new avenues in chemical synthesis, with applications anticipated for more complex molecules and drug candidates. Cooperative catalysis, combining light and metal catalysts, embodies a sustainable chemical process. This study is a major step towards creating an environmentally harmonious future society.”
###
The article, “Enantioselective Heterocoupling of 2-Naphthylamines with 2-Naphthol Derivatives via Cooperative Photoactivation and Chiral Vanadium(V) Catalysis,” was published in ACS Catalysis at DOI: https://doi.org/10.1021/acscatal.5c05038
About The University of Osaka
The University of Osaka was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan’s leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world. Now, The University of Osaka is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Enantioselective Heterocoupling of 2-Naphthylamines with 2-Naphthol Derivatives via Cooperative Photoactivation and Chiral Vanadium(V) Catalysis
Article Publication Date
17-Oct-2025
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

AloJapan.com